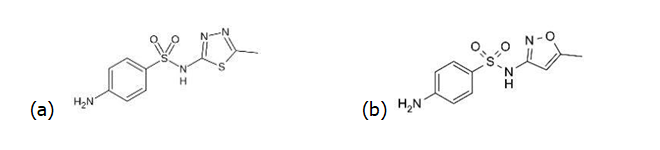- Case StudyHelp.com
- Sample Questions
Medicinal Chemistry Coursework Assignment Solution
Case Study Help offers you top quality medicinal chemistry assignment help. Our team of experts assures 100% academic success for all university students at the best price. Hire our assistant writer for your assignment writing service and score an A+ grade. Feel free to choose our services, and we pledge to do your assignment on time.
- Topic :: Medicinal Chemistry
- Number of Words*: 1500
- Citation/Referencing Style: Havard
Your coursework consists of two parts, A and B, which are equally weighted and given below. Your total mark for this coursework will contribute 50% to your final module mark. Submit your work as a single Word file. We cannot ensure that other formats than Word are compatible with markers’ software or guarantee to mark incorrect formats. It is your responsibility to submit the coursework in the format stipulated above. Your marks may be affected if your tutor cannot open or properly view your submission.
Answer ALL the questions in both parts A and B below within a single Word file by typing in your answers to each question and scanning in original drawings or a spectrum where appropriate. You may draw chemical structures neatly by hand and scan them into your submission. Please save your answer file with your student number as the title and use Arial 12 point font and 1.5 line space for your typed answers.
This is an individual assessment and your answer must be an independent piece of work, written in your own words. There is a maximum word count limit of 1500 +/-10% on this assignment. Anything after the maximum limit will not be marked. Do not directly copy and paste work from any other source, including material supplied on Blackboard, or work with any other person. Text-matching, plagiarism detection software will be used on all submissions; this compares your work with Blackboard, other students’ submissions, as well as external sources. Do not be tempted to contact others for this assessment. Sharing work or colluding is an assessment offence. Students found to have committed an assessment offence will be managed according to the assessment offence policy.
You must submit your assignment before the stated deadline of 2pm Tuesday 16th April 2024 (week 35) by electronic submission through Blackboard. Multiple submissions can be made to the portal before the deadline, but only the final one will be accepted. Please save your work frequently. Do not leave submission to the very last minute, allow time in case of technical issues.
The date and time of your submission is taken from the Blackboard server and is recorded when your submission is complete, not when you click Submit. It is essential that you check that you have submitted the correct file(s), and that each complete file was received. Submission receipts are accessed from the Coursework tab.
Part A Instructions: The following questions relate to the antibiotic sulphonamide drugs and to their derivatives. Answer ALL questions below by showing your calculation in question 1, giving the correct answers to questions 2-4 and 6-7, and naming and drawing the chemicals required in steps (a)-(d) for question 5.
Also Read: Synthesis of Lidocaine a Medicinal Chemistry Assignment Answers
Questions 1- 4 refer to your lab synthesis of sulfathiazole
- Assuming 3.9 g of sulfathiazole had been isolated from your experiment, what would be the resulting percentage yield? Show your calculation in full.
- For the synthesis of 4-acetamidobenzenesulfonyl chloride 5, why would aniline (C6H5NH2) not be appropriate as the starting material?
- What is the function of K2CO3 in the formation of sulphonamide 7 in step a?
- Why is it important to dry both the solvent and glassware for step a?
- Discovery of the antibiotic sulphanilamide led to rapid development of a large number of structurally related sulphonamides. Some of these were useful leads to compounds with other medicinal properties. Amongst these, sulfasalazine was active in the treatment of ulcerative colitis, a potentially fatal disease of the colon.
As a medicinal chemist, you are about to carry out the synthesis of sulfasalazine, starting from aniline. Give the chemical names and draw the chemical structures of the reagents you will need to use for the all the steps marked (a)-(d).
1. Step (a) in question 5 above results in the para product only. Explain why this occurs.
2. Identify the bioisosteres that have been used to develop each of the following drugs from sulfathiazole;
Part B: Instructions Answer ALL the following questions.
Also checkout: CHEM1500 Chemical Bonding and Organic Chemistry Assignment Answers
Questions 1- 7 refer to your lab synthesis of lidocaine.
- Scan into your Word document a suitably labelled IR spectrum of the intermediate, identifying the two peaks associated with the amide functional group.
- Give the weight of intermediate product made, and give your full calculation of the percentage yield obtained.
- Why would ethanol be a poor choice of a solvent for the reaction between 2,6- dimethylaniline and chloroacetyl chloride in stage 1 of your synthesis of lidocaine?
- In stage 1 of your synthesis of lidocaine, which one of (a)-(d) is true of the reaction between the primary amine group of 2,6-dimethylaniline and chloroacetyl chloride?
- The acyl carbon is less electrophilic than the alkyl carbon so reacts
- The acyl carbon is more electrophilic than the alkyl carbon so reacts
- The primary amine is too electrophilic to react with the alkyl
- The acyl and alkyl carbons are equally
- Explain why chloroacetyl chloride doesn’t attack chloro-2,6-dimethylacetanilide in stage 1 of your synthesis of lidocaine.
- In stage 2 of your synthesis of lidocaine, which one of (a)-(d) best explains why hydrochloric acid protonates the nitrogen atom of the diethylamine group of lidocaine preferentially to that of the amido group?
- The amide nitrogen is protected by the two methyl groups of the aromatic
- The diethylamine nitrogen has a lower pKa than the amide
- The two ethyl groups are electron
- The amide nitrogen has a lower pKa than the diethylamine
- Give both the chemical name and chemical formula of the by-product that is washed away at the sink in step 2 of the synthesis of lidocaine.
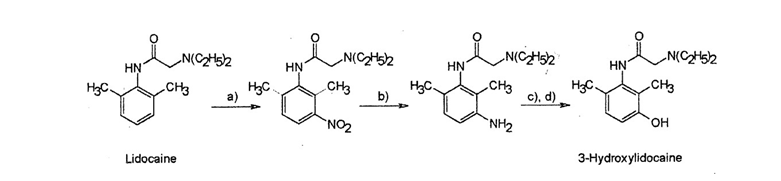
- Illegal doping of horses by administration of lidocaine can be detected by analysis of urine for the metabolite, 3-hydroxylidocaine. This compound can be made synthetically, as shown below, for use as a standard in the analytical method.
Name the reagents required to carry out step a) and explain why the nitro group adds to the ring adjacent to the methyl group.