- Case StudyHelp.com
- Sample Questions
Looking for Synthesis of Lidocaine a Medicinal Chemistry Assignment Answers? Get Case Study Answers on Synthesis of Lidocaine a Medicinal Chemistry Coursework. We Provide Chemistry Assignment Help, Science Assignment Writing Service & Assignments Writing Help from Masters and PhD Expert at affordable price? Acquire HD Quality research work with 100% Plagiarism free content.
Medicinal Chemistry Assignment Details:
Topic: Lidocine synthesis
Document Type: Coursework
Subject: Chemistry
Deadline:*: As Per Required
Number of Words: As Per Required
Citation/Referencing Style: As Per Required
How Can We Write Chemistry Assignments with Accurate Solutions?
It multiple choice questions there no writing I just need write answers for my questions in medicinal chemistry
MEDICINAL CHEMISTRY
USSK5B-15-2
Experiment 2: Synthesis of Lidocaine A Introduction
Lidocaine (below right) is the common name of an important local anaesthetic which is also effective in the treatment of arrhythmia (erratic heart beat). Incorporation of a 2,5-dimethyl substituted aromatic ring adjacent to the amide in lidocaine renders it less subject to attack by metabolising enzymes (amidases), and increases residence time of the drug within the body. This protecting structural feature is known as a steric shield.
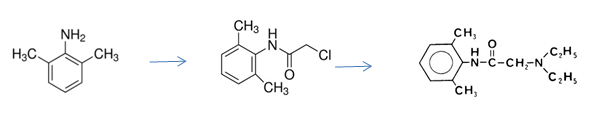
In this experiment you will start the preparation of lidocaine by converting 2,6-dimethylaniline to N-(2,6-dimethylphenyl) chloroacetamide, using - chloroacetyl chloride, ClCH2COCl. Addition of excess diethylamine to this intermediate gives the final product, lidocaine. Acid used in both stages of this preparation forms salts of the intermediate and product, so there are various stages of pH control and/or extraction before finally isolating the product.
Work in pairs to complete both stages of the synthesis during the first of the two, three hour sessions given on your personal timetable. The weights, mpts and IR spectra will be recorded in the second of these weeks. Assessment information is given on the last three pages and should be completed individually.
B Experimental
All parts of this experiment should be carried out within a fume cupboard.
Stage 1:
- Place a 250 mL conical flask containing a white stirrer bar and thermometer on a flat plate stirrer, and then add in the following order to the stirred flask; 7.50 mL 2,6 dimethylaniline, 25 mL glacial acetic acid and 5.00 mL -chloroacetyl chloride. The temperature of this mixture will rise to 40-50 °C as the reaction proceeds.
- After 5 minutes, gradually add an aqueous solution of sodium acetate trihydrate (5 g/100 mL provided) to the stirred mixture. Cool the flask on an ice bath for 5 minutes to about 20 °C, and collect the solid formed by vacuum filtration using a Buchner flask and funnel. (Use the filtered liquid to rinse the reaction flask to help transfer all the solid onto the paper.)
- Discard the liquid, and rinse the solid on the filter paper with 25mL de-ionised water, then 10mL methanol. Remove the stirrer bar and leave the solid drying for 5 minutes on the filter while the vacuum source is still attached. Clear away the conical flask, heater and ice bath whilst you wait.
- Place the solid on a watch glass in an oven at 110 °C for 10 minutes whilst you clear away the filtration equipment from this step and set up the reflux equipment for the next step. Place the solid in a labelled sample bottle and submit to a member of staff.
- Record the weight of this crude intermediate, the melting point range (starting from about 110 °C) and the infrared spectrum of the product (also known as -chloro-2,6-dimethylacetanilide).
Note: All reagents and equipment used in the next section must be dry.
Stage 2:
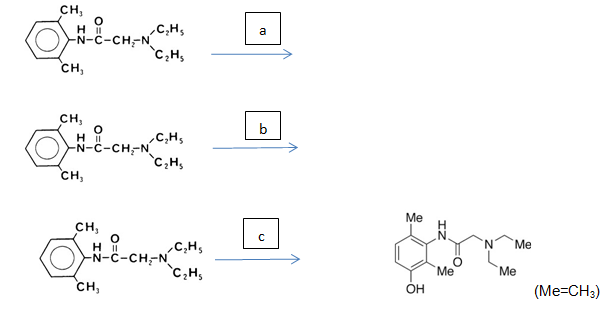
- Place 4.8 g of N-(2,6-dimethylphenyl) chloroacetamide (provided dry) in a 100 mL one neck, round-bottomed flask with a small white stirrer bar, and add 25 mL of dry xylene, followed by 9 mL diethylamine. Equip the flask with a reflux condenser and bring the stirred reaction mixture to a vigorous reflux on a rotamantle. (Solids should be in solution after a few minutes at reflux.) After 20 minutes, combine approx. equal volumes of salt and ice in an ice bath, and rapidly cool the flask to about room temperature.
- Filter off the crystalline solid that forms by vacuum filtration, and transfer the liquidfiltrate to a 100 mL separating flask. (The solid is water soluble and can be washed down the sink with plenty of water. What is this solid?)
Safe use of the separating flask will be demonstrated.
- Shake the liquid filtrate with 25 mL 3M HCl in the separating flask, and allow the liquid mixture to settle for a couple of minutes. Two layers should form – if not consult a member of staff. Transfer the lower aqueous layerto a 250 mL conical flask, and discard the upper organic layer into the waste container provided.
- To the acidic aqueous extract in the conical flask, carefully add with swirling about 10 mL 8M KOH solution (provided, care corrosive) to make the mixture strongly basic to Universal indicator pH paper. You should see a thin layer of yellow oil in the flask.
- Place the flask in an equal mixture of salt and ice for about 10 minutes whilst you clear away the equipment from the filtration and extraction. The oil should now have become a solid, but if not, gently scratch the inside of the flask with a glass rod until crystallization occurs.
- Collect the crude solid by vacuum filtration. Gently break up the solid into a powder using the back of a spatula, and allow the solid to dry under vacuum for a few minutes. Transfer the solid to a labelled sample bottle, cover the mouth with a filter paper, and secure the paper with a rubber band. Submit this sample bottle to a member of staff.
- In the following session record the weight of this crude lidocaine product and record the IR spectrum.
Assessment material for this practical is given on the following pages.
MEDICINAL CHEMISTRY 2019-20 ASSESSED COURSEWORK WORKSHEET 2
Your mark for this piece of work will constitute 50% to your final coursework mark for the module. Complete all the following questions as instructed, staple the pages to your coversheet with answers to the sulphonamide worksheet 1, and submit via the box on the Coursework Hub.
Instructions: The following questions relate to the synthesis and metabolism of lidocaine and its derivatives. Answer ALL questions on these pages in pen by ticking the correct answer letter or by completing the answers to questions 4 and 7-9. Any answer in pencil or notclearly given will not be marked. Maximum marks are shown in brackets for each part.
1. Submit both the IR spectra from your practical work, each clearly labelled and identifying the peak due to the amide carbonyl group. On the back of each spectrum give the relevant weight of product and a full calculation of percentage yield, and give the melting point range for the intermediate. (10)
Answer ALL the following, clearly marking the correct answers to each of questions 2, 3 and 5-7, and completing the answers to questions 4 and 7-9.
2. Why would ethanol be a poor choice of a solvent for the reaction between 2,6dimethylaniline and -chloroacetyl chloride in stage 1 of your synthesis of lidocaine?
a). It reacts with the primary amine group of dimethylaniline.
b). The product will react with ethanol.
c). It reacts with the alkyl chloride group of -chloroacetyl chloride.
d). It reacts with the acyl chloride group of -chloroacetyl chloride.
3. In stage 1 of your synthesis of lidocaine, which one is true of the reaction between the primary amine group of 2,6-dimethylaniline and -chloroacetyl chloride?
a). The acyl carbon is less electrophilic than the alkyl carbon so reacts preferentially.
b). The acyl carbon is more electrophilic than the alkyl carbon so reacts preferentially.
c). The primary amine is too electrophilic to react with the alkyl carbon.
d). The acyl and alkyl carbons are equally reactive.
4. Using an appropriate diagram, explain whychloroacetyl chloride doesn’t attack chloro-2,6-dimethylacetanilide in stage 1 of your synthesis of lidocaine.
5. In stage 2 of your synthesis of lidocaine, which one best explains why hydrochloricacid protonates the nitrogen atom of the diethylamine group of lidocaine preferentially to that of the amido group?
a). The amide nitrogen is protected by the two methyl groups of the aromatic ring.
b). The diethylamine nitrogen has a lower pKa than the amide nitrogen.
c). The two ethyl groups are electron withdrawing.
d). The amide nitrogen has a lower pKa than the diethylamine nitrogen.
6. Correctly identify whether each of the following statements about local anaesthetic agents is true (T) or false (F).
a). The amide nitrogen must always be adjacent to the aromatic ring.
b). A secondary or tertiary amine is an essential part of the pharmacophore for local anaesthesia.
c). The –CH2– between amide and amine can be extended to –C2H4-.
d). With the exception of the steric shield and amide chain, the aromatic ring must not be further substituted.
7. Give both the chemical name and chemical formula of the by-product that is washed away at the sink in step 2 of your synthesis of lidocaine.
8. For each of the following steps(a)-(c) in the metabolic pathway of lidocaine, correctly name the biochemical process occurring and identifying whether it is a phase I or phase II process.
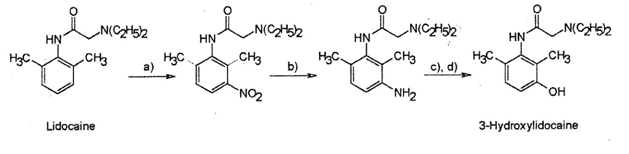
9. Illegal doping of horses by administration of lidocaine can be detected by analysis of urine for the metabolite, 3-hydroxylidocaine. This compound can be made synthetically, as shown below, for use as a standard in the analytical method.
- Give the two reagents required to carry out step a), and draw a mechanism for this step. (4)
- In step a), why does the nitro group add to the ring adjacent to the methyl group? (1)
Reference ID: #getanswers2001147